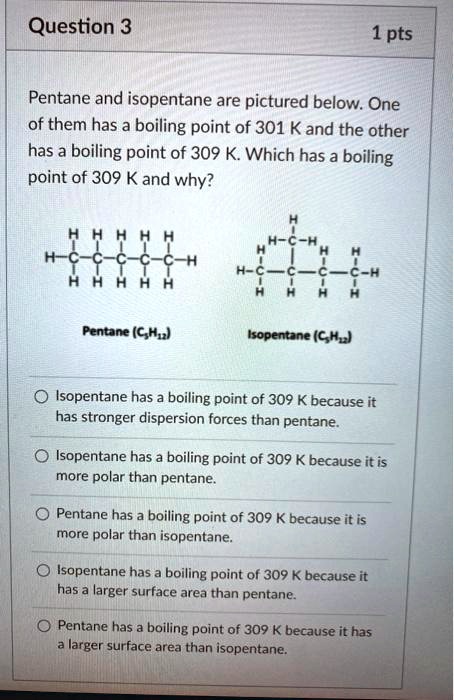
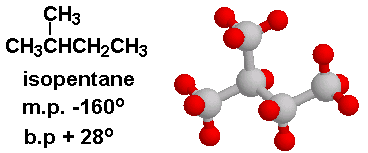
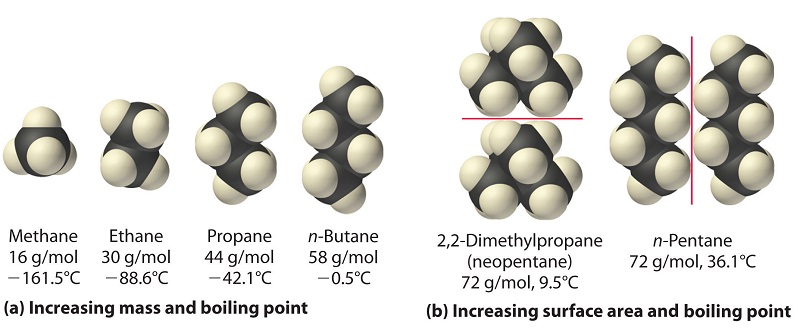
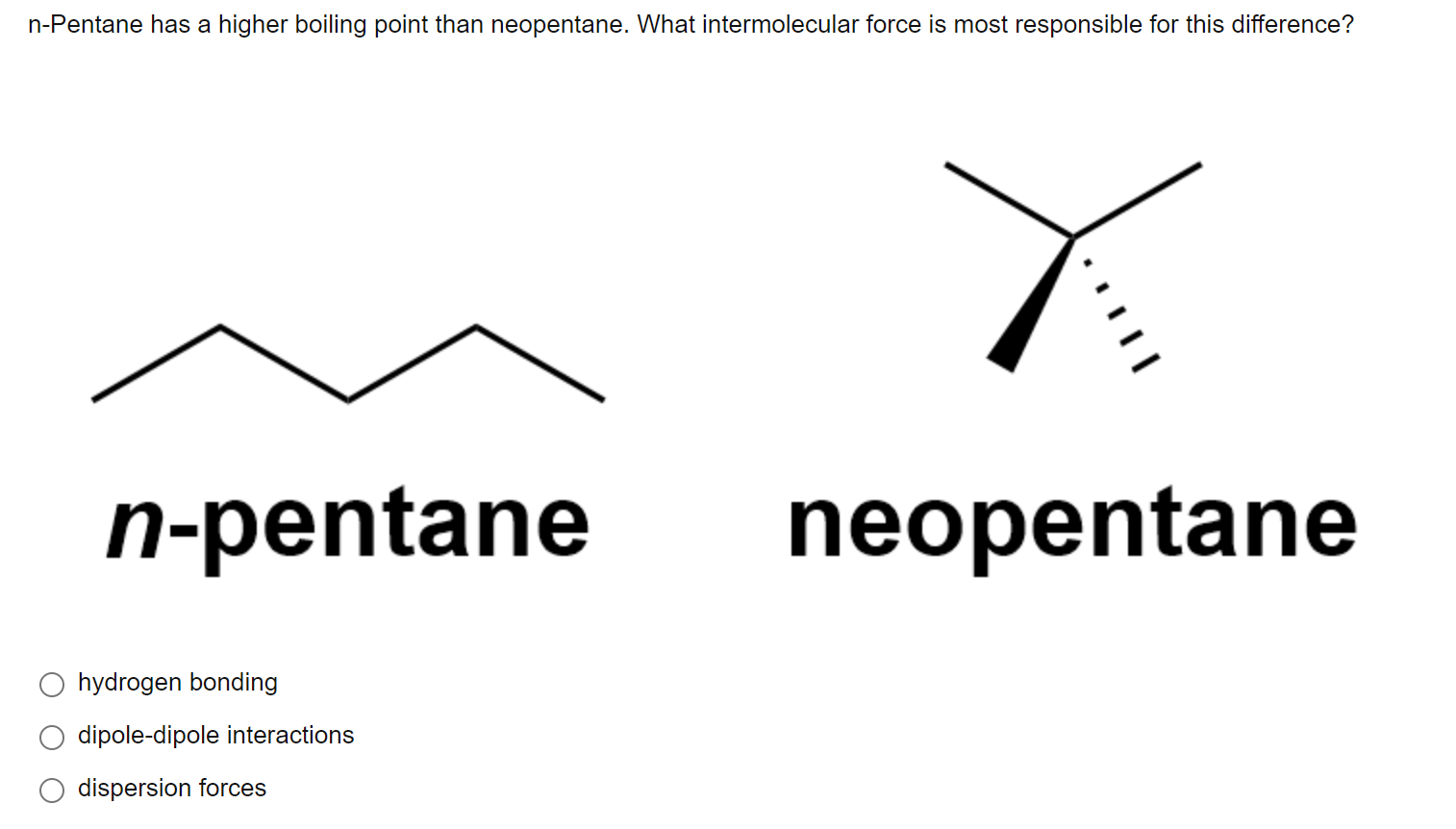
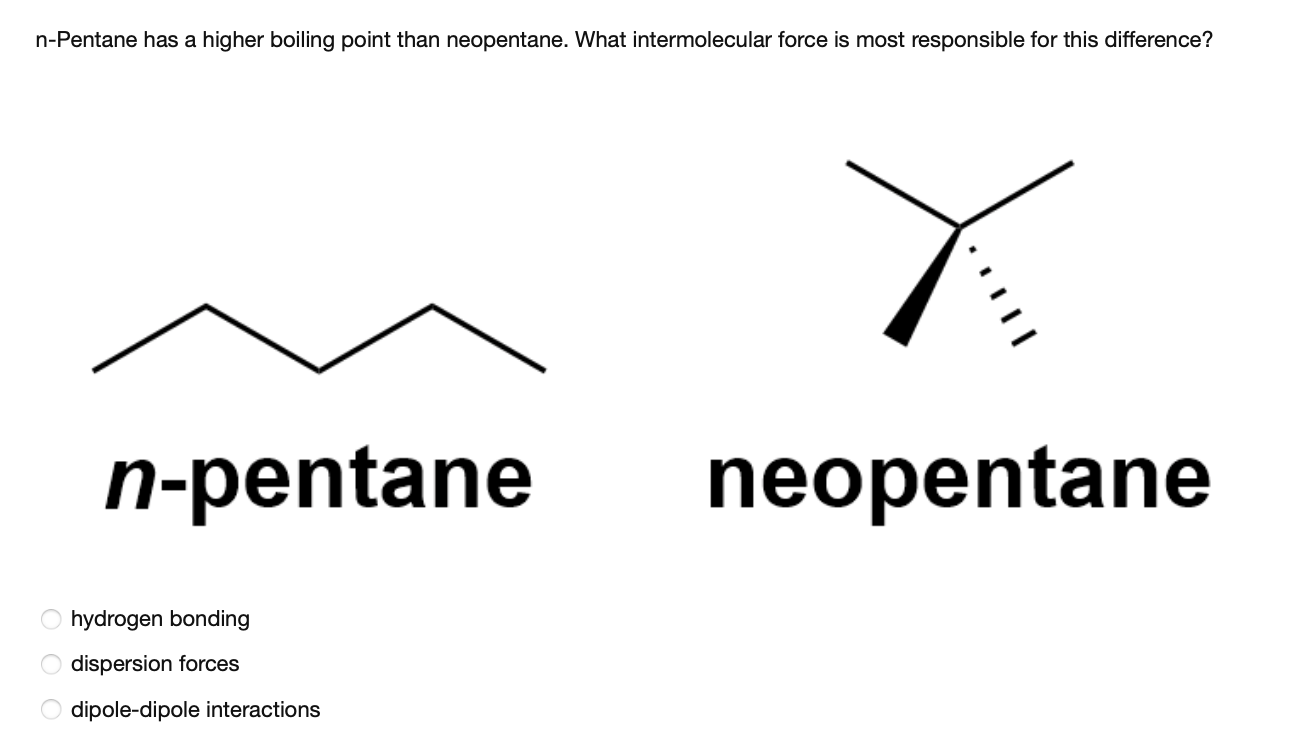
Explain why n-pentane has higher boiling point than neo pentane but melting point of neo-pentane is more than n-pentane

why neopentane has higher melting point than n pentane - Chemistry - Chemical Bonding and Molecular Structure - 13416933 | Meritnation.com

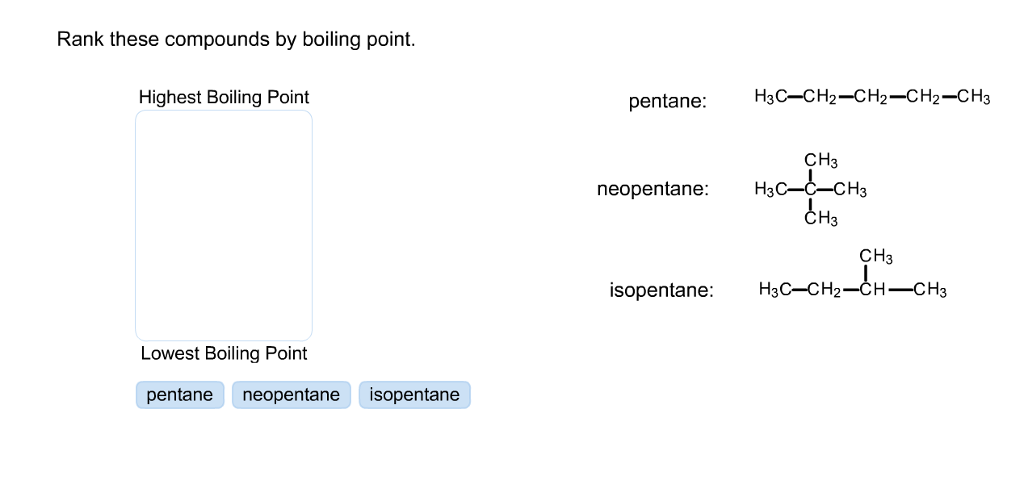
Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange
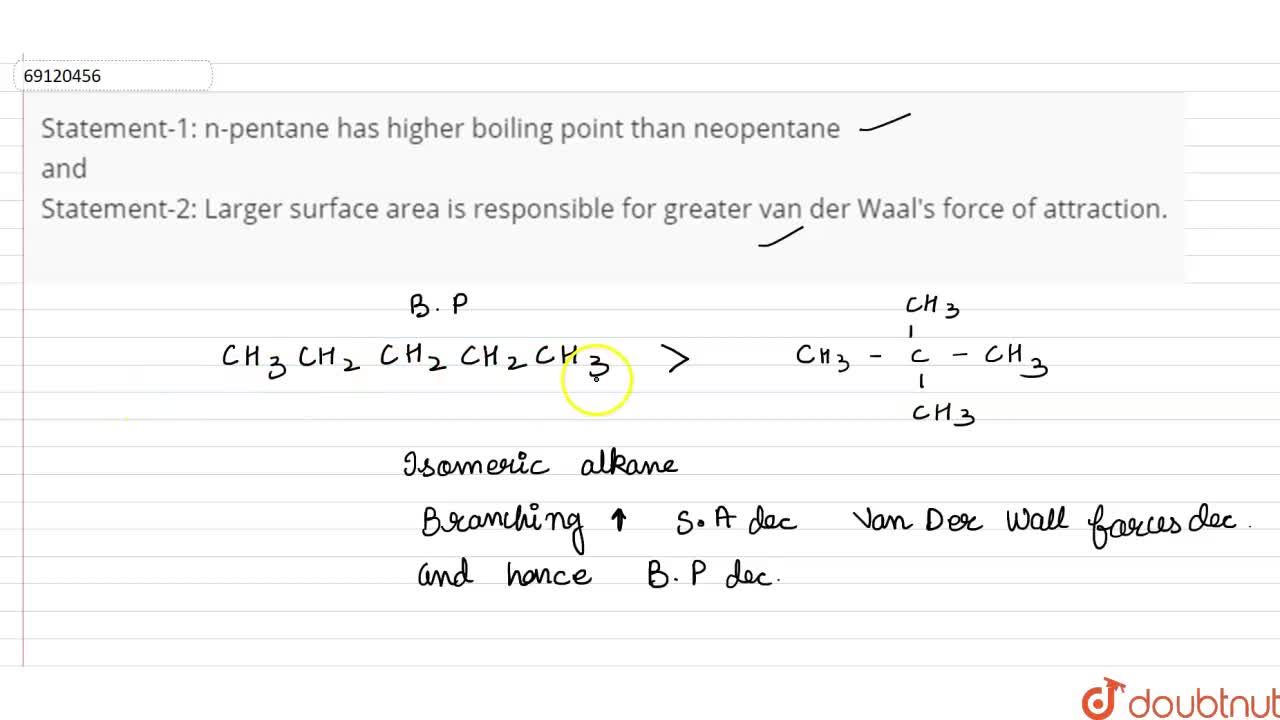
Statement-1: n-pentane has higher boiling point than neopentane and Statement-2: Larger surface area is responsible for greater van der Waal's force of attraction.

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane

Melting And Boiling Point Npentane Nhexane Stock Illustration - Download Image Now - Acid, Alcohol - Drink, Atom - iStock